Abstract
Introduction: Children with Down Syndrome (DS) have a 20-fold increased incidence of acute lymphoblastic leukemia (ALL) and a 70% higher risk of relapse than children with ALL and no DS. Treatment for DS children with relapsed ALL is challenging due to the high treatment-related mortality associated with both intensive chemotherapy and hematopoietic stem cell transplantation (HSCT) as well as high rates of second relapse. Outcomes for relapsed ALL in DS are dismal, with recent studies citing an event-free survival (EFS) as low as 17%. Alternative treatment approaches are needed for this patient population. Treatment with anti-CD19 chimeric antigen receptor T-cells (CARTs) have shown high remission rates in patients with multiple relapsed/refractory ALL with an acceptable safety profile. CARTs are generally not indicated for first relapse of ALL; DS-specific indications for CARTs do not exist. In Ontario, due to the specific susceptibility of children with DS-ALL, CARTs were made available to this population upon first relapse. We describe the population-based Canadian experience using CARTs for first relapse of DS-ALL.
Methods: Efficacy and safety data on all children referred for CARTs at the Hospital for Sick Children, in Toronto, Canada were retrospectively collected in a central database and validated by treating clinicians. Here we report all patients with DS suffering a first relapse of ALL at age <18 years referred for commercially available CARTs (tisagenlecleucel) between 2019 and 2021.
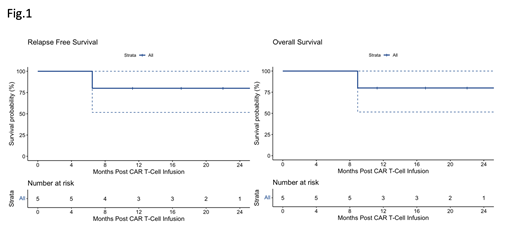
Results: Five patients with DS received tisagenlecleucel for first relapse of ALL during the study period. Median age at original diagnosis was 6.3 years (range 3.4-11.5). Four patients presenting with National Cancer Institute (NCI) standard risk and one with NCI high-risk disease were initially treated on Children's Oncology Group based protocols. Four patients presented with relapse >36 months from diagnosis and one patient with early relapse. Mononuclear cells were successfully collected for all patients on the first attempt. Patients were bridged from collection to infusion with low intensity chemotherapy regimens. Tisagenlecleucel was successfully manufactured for all patients and infused at a median of 69 days (range 56-101) from collection of T cells. Four patients developed CRS: two patients grade 1, one patient grade 2, and one patient grade 4 requiring ICU care and treatment with tocilizumab and steroid. All patients achieved minimal residual disease (MRD) negative remission one month after infusion, with B-cell aplasia. Two patients showed early (< 6months post infusion) B-cell recovery consistent with CAR T-cell loss; both received off-label reinfusions of tisagenlecleucel and again achieved B-cell aplasia. One patient had a seizure with the first Fludarabine dose of lymphodepleting (LD) chemotherapy for re-infusion and was found to have elevated intracranial pressure. After treatment with Topiramate and anti-seizure prophylaxis the patient tolerated LD chemotherapy without further neurological events. No patient received further ALL therapy; none went on to HSCT. One patient suffered a CD19-negative relapse 7 months post infusion and was transitioned to palliative-intent treatment. Two patients developed prolonged neutropenia with one patient showing recovery (ANC>1x10E9/l) at 5 months post infusion and the patient who had relapsed disease remained neutropenic until relapse. Infectious complications in the first 6 months post infusion were rare. One patient, who was not neutropenic, required two hospital admissions for line related sepsis. With a median follow-up time of 519 days (range 197-785), the 1-year estimated EFS and overall survival were both 80% (95 th confidence interval 52-100%; Figure 1).
Conclusions: Tisagenlecleucel was safe and effective in a small cohort of children with DS and first relapse of CD19+ ALL, with achievement of long-term remissions without further therapy, and an EFS superior to that seen in historical cohorts. Rate of acute complications were not increased compared with reported outcomes in patients without DS. Further evaluation of tisagenlecleucel in this setting is warranted.
Krueger: KITE/Gilead: Consultancy; Novartis: Consultancy; SOBI: Consultancy. Whitlock: Sobi Pharmaceuticals: Consultancy; Novartis: Research Funding; Amgen; Jazz Pharmaceuticals: Honoraria. Gupta: Jazz Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees.
tisagenlecleucel for early B-cell recovery

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal